Not known Factual Statements About Proleviate includes FDA-approved Ingredients
To treat relapsed or refractory mantle cell lymphoma in Grown ups that have had at the least two traces of systemic therapy, which includes a BTK inhibitor
No less than 75 days before introducing such a dietary health supplement into interstate commerce or providing it for introduction into interstate commerce, the maker or distributor should submit a notification to FDA with the data on The premise of which the company has concluded which the NDI-containing dietary health supplement will reasonably be anticipated being Protected. Additionally, FDA’s regulations involve people who manufacture, bundle, or hold dietary dietary supplements to observe present great manufacturing practices that help ensure the identification, purity, quality, power, and composition of dietary dietary supplements. FDA normally will not approve dietary health supplement promises or other labeling prior to use.
This database presents info on the utmost potency for each device dose of excipient in authorized drug merchandise in The usa for a certain route of administration.
During the period 1980–2022, there was a considerable rise in the quantity of advertising approvals of recent drug goods, especially biologics, with the majority currently being antineoplastic and immunomodulating agents. An important proportion of the newly authorized medicine were being granted acceptance through designations and expedited assessment methods, which will not involve the demonstration of addressing unmet health care needs or furnishing superior client Gains compared to existing marketed alternatives.
Since the law prohibits the distribution and sale of adulterated dietary nutritional supplements, makers and distributors have initial responsibility for making certain that their dietary supplements meet up with the protection standards for dietary dietary supplements. When brands and distributors usually do not satisfy that duty and adulterated dietary supplements attain the industry, FDA has authority to implement the legislation to protect shoppers. Generally, FDA is restricted to postmarket enforcement due to the fact, in contrast to medication that needs to be tested Protected and powerful for his or her intended use right before promoting, there isn't any provisions in the law for FDA to approve dietary health supplements for security right before they reach The customer.
was combined with both small-dose vancomycin or metronidazole. Based on the 2010 tips for management of CDI in Grown ups, printed jointly through the Society of Healthcare Epidemiology of The usa and the Infectious Disorders Modern society of The us, no compelling proof exists to guidance program usage of probiotics for prevention or remedy of CDI (
Very well, Easter has come, which can only mean that it is time for our spring holiday getaway compliance Exclusive!
To use as Element of a treatment method program for recently diagnosed acute myeloid leukemia that satisfies specified standards
A route of administration is a method of administering a drug to some web page in a individual. An extensive list of unique routes of administration can be found Proleviate includes FDA-approved Ingredients over the FDA Structured Product or service Labeling (SPL) Web content under terminology.
The mission with the FDA includes protecting and selling public health by guaranteeing the security and efficacy of medication and biological products. The FDA can also be accountable for advancing general public health by “helping to velocity innovation”one.
We also don’t know just how much on the probiotic individuals must just take or who'd be probably to learn. Even to the ailments which have been analyzed by far the most, scientists are still Operating toward locating the solutions to these inquiries.
Reality #nine: Applying CBD raises security considerations, and several CBD items are being offered with unproven promises saying they might treat or prevent disorders or situations.
). A dietary dietary supplement is outlined from the Dietary Supplement Health and Education Act (DSHEA) of 1994 as a product taken by mouth that contains a “dietary component” meant to health supplement the diet program. Health supplements should contain >1 of the subsequent dietary ingredients: a vitamin; a mineral; an herb or other botanical (excluding tobacco); an amino acid; a dietary material for use by people to complement the food plan by expanding the full dietary intake; a concentrate, metabolite, constituent, extract; or blend of any of the above mentioned (
The https:// makes certain that you will be connecting for the official Internet site Which any information you present is encrypted and transmitted securely.
 Michael C. Maronna Then & Now!
Michael C. Maronna Then & Now!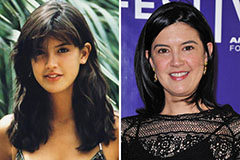 Phoebe Cates Then & Now!
Phoebe Cates Then & Now!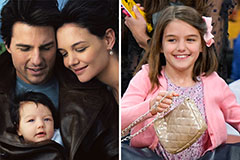 Suri Cruise Then & Now!
Suri Cruise Then & Now! Barbara Eden Then & Now!
Barbara Eden Then & Now! The Olsen Twins Then & Now!
The Olsen Twins Then & Now!